Little Sparrows Technologies partners with Fathom Optics to protect its latest product launch
Little Sparrows Technologies was launched in 2013 by Donna Brezinski, MD, and Gary Gilbert, MD, both physicians affiliated with Harvard Medical School. Its initial product, bili∙hut™, was created to provide the most clinically effective phototherapy treatment available to treat infant jaundice, delivering NICU-intensity care at the mother's side in the hospital or home.
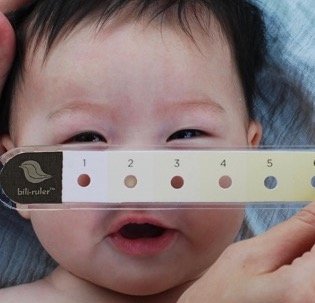
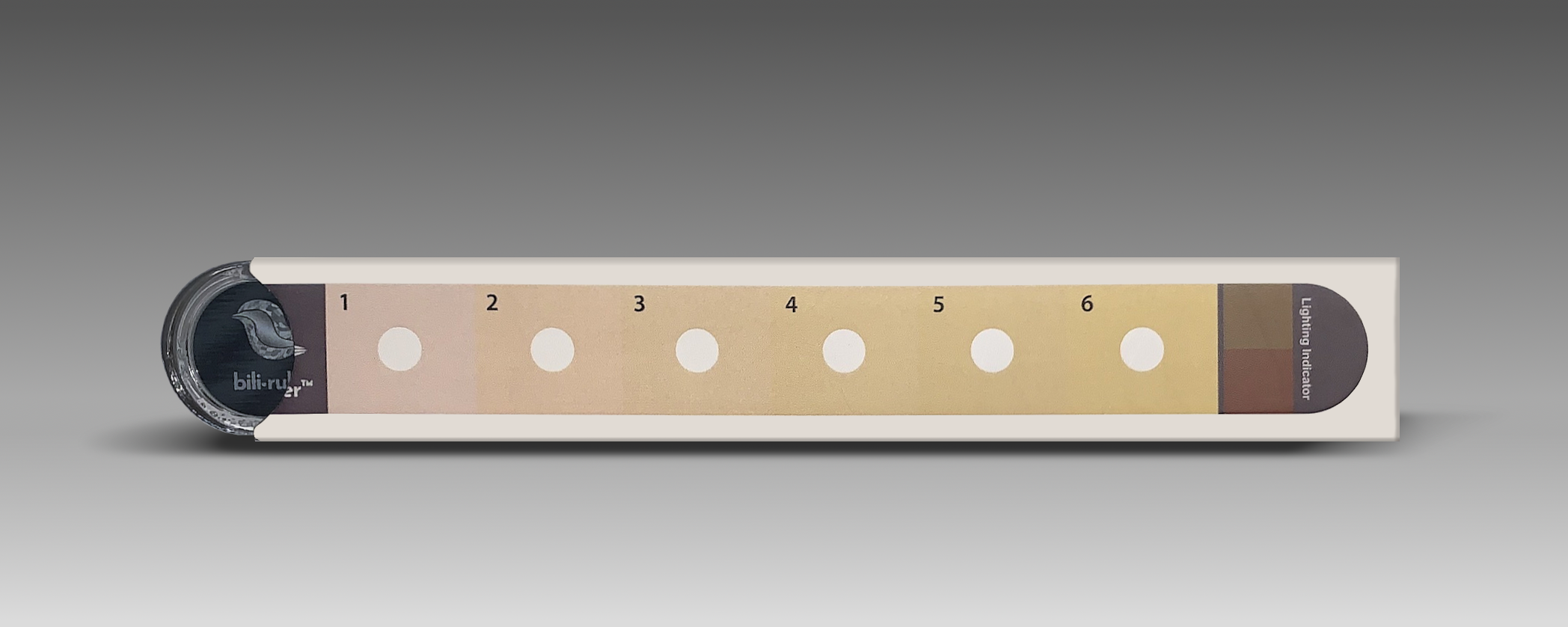
Its second product, bili∙ruler™, is a tool to aid in the visual assessment of infant jaundice and was developed based on research by Dr. Anne CC Lee and the Global AIM Lab at Brigham and Women’s Hospital as a part of the “Saving Lives at Birth” grant funded by USAID and the Gates Foundation. The original inspiration for the device was a research tool developed in the 1950s for the visual diagnosis of jaundice, the Gosset icterometer. This early tool had multiple limitations, including non-standardized reference colors and high production expense. Though conceptually similar, the bili∙ruler™ expanded on this idea, creating a commercially viable, standardized device that was more user-friendly using modern color processing techniques and visual, human-centered design. The result is a versatile device that can be used in the field to screen infant jaundice, even in remote locations without access to mobile networks or electricity.
The Challenge
Given the range of environments where the tool would be used, Little Sparrows Technologies was also concerned about potential knockoff devices that could pose a high risk to patients. In particular, the company was seeking a solution for product authentication that did not require mobile phones or access to electricity. Product engineer Nolan Smith explains, “We wanted to reassure end users that they have an original and validated device without requiring an electronic device for authentication. We also wanted to dissuade bad actors from creating a facsimile and violating our intellectual property.”
As it turns out, Little Sparrows Technologies is one of many biomedical companies to face such challenges. Much has been reported about the counterfeiting of anti-malarials in Africa, and more recently about the counterfeiting of COVID certificates around the world. In both of these cases, the solution is not as simple as merely providing digital authentication such as a QR code, since scanning of these codes requires the availability of a connected device. This also requires overcoming the difficulties that can be associated with taking a scan in many social settings.
The Solution
To meet these challenges, Little Sparrows Technologies used a different, more creative approach. It partnered with Fathom Optics, with whom it had shared start-up space in Boston for several years. Fathom’s patented software uses light field technology to bring 3D and motion graphics to printing and packaging without requiring special inks, substrates, or additional materials such as lenticular sheets or metallic foils. Fathom’s platform generates moiré-like effects, and can be used to create a customized optically-varying (OV) authentication feature that looks visually unique relative to any other product in the market.
Best of all, Fathom’s embellishments are generated for the specific press that they’ll be run on, further enhancing the security of the printed device. Fathom built its expertise in this regard by carefully characterizing many dozens of printing presses at prominent label and packaging production sites to understand how they can robustly add depth, motion and chromatic effects to a wide range of print applications including prime labels, shrink sleeves, and point-of-purchase displays. It used this knowledge to provide a more secure authentication solution for Little Sparrows Technologies.
Little Sparrows Technologies first turned to Fathom for help in June 2020. According to Tom Baran, CEO and co-founder, “When Little Sparrows Technologies came to us with this challenge, we knew they’d be interested in conveying the authenticity of their product using a customized design that was both distinctive and visually engaging but at the same time not too busy, and not ‘just metallic’. So, we got to work iterating with Nolan, Donna and the team there with whom [Fathom co-founder / CTO] Matt Hirsch and I had developed a great relationship over the past years. Nolan and Donna encouraged us to push the boundaries of the technology and of design, which was fantastic.”
In Production
Amherst Label production facility in Milford, NH.
To produce and integrate the optically-varying feature, Fathom and Little Sparrows Technologies partnered with Amherst Label, a family-owned converter that has operated for over 40 years in New Hampshire. Amherst was an early certified adopter of Fathom’s effects, originally engaging with the company on an R&D project to see if there was an opportunity for its customers, and to make sure its capabilities and press worked with the Fathom technology. It worked so well that the converter ended up running a variety of different jobs, including promotional pieces in spaces ranging from beer to personal care.
Nye Horner, Amherst Label
Little Sparrows Technologies provided Amherst with the product components, proprietary color strips which had been printed on a wide-format printer. Amherst applied the Fathom feature depicting a moving, stylized Little Sparrows Technologies’ logo, using a permanent adhesive on a layflat 2 mil polypropylene material. The feature itself was produced with 1-bit plate images that Fathom had developed specifically for Little Sparrows Technologies, and that were produced nearby at Concord Engraving using MacDermid ITP60 plates. Amherst then die-cut the full ruler and it was finally encased in plastic.
Nye Hornor, President of Amherst Labels summed it up: “The best thing about the technology that Fathom has developed is that you get the ‘lenticular’ effect without the cost of using the true “lenticular” technology. Also, the ability to have a security feature or capability which makes a product unique is great to be able to offer to our customers and prospects.”
Results
The production bili-ruler™ with Fathom authentication feature printed in the logo.
“I would highly recommend Fathom Optics to any medical device company looking to improve their product’s security. We can have custom authentication features that look different from anything else.”
As the product is gearing towards international launch, preorders for the bili∙ruler™ have come from areas as diverse as Canada, Costa Rica, Ghana and Nepal. Little Sparrows Technologies is also seeking FDA clearance of the device to enable easier screening of jaundiced babies in the US, reducing the need for repeated blood testing for jaundice. With the current challenges posed by COVID-19, point-of-care screening tools such as bili∙ruler™ will help mothers and babies prevent unnecessary exposure from a hospital visit.
Smith concludes, “I would highly recommend Fathom Optics to any medical device company looking to improve their product’s security through custom-designed labels.”
Baran adds, “Nolan and Donna at Little Sparrows Technologies were a joy to work with, and it was wonderful to help them with the specific authentication challenges that were unique to their product. We also couldn’t have done it without Nye and the team at Amherst, who did a really fantastic job—and high-quality work—taking this project from concept to production-scale execution.”
How bili∙ruler works
Designed with a series of yellow swatches of increasing intensity that correlate to ranges of serum bilirubin levels and bili∙ruler™ helps determine the severity of jaundice.
Using the color that most closely matches a baby’s blanched skin color on the tip of the nose, the user can approximate the infant’s bilirubin level and determine the need for further diagnostic testing and treatment of jaundice.
Bili∙ruler™ has a strong positive correlation with both transcutaneous and serum bilirubin measurements. It was validated on nearly 800 multi-ethic babies demonstrating equivalency to hospital devices (transcutaneous bilirubinometers) which cost thousands of dollars.